Abstract

Background: Cold agglutinin disease (CAD) is a rare chronic autoimmune hemolytic anemia that is mediated by classical complement pathway activation, leading to fatigue and poor quality of life (QoL). In the randomized, double-blind, placebo-controlled Part A of the Phase 3 CADENZA trial (NCT03347422), treatment with sutimlimab - a C1s complement inhibitor - rapidly halted hemolysis, increased hemoglobin levels and improved fatigue in CAD patients with no recent history of transfusion. Rapid improvements compared to placebo were also observed for patient-reported outcomes (PROs) up to 26 weeks.
Aim: To report the long-term effect of sutimlimab treatment on patient-reported outcomes in patients with CAD from the open-label Part B extension of CADENZA.
Methods: In the open-label extension Part B, all patients who had completed Part A were eligible to receive biweekly doses of sutimlimab at 6.5 g (if <75 kg) or 7.5 g (if ≥75 kg), continuing until 1 year after the last patient completed Part A. PRO endpoints included Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue), 12-Item Short Form Health Survey (SF-12), EuroQol visual analogue scale (EQ-VAS), Patient Global Impression of Change from baseline (PGIC), and Patient Global Impression of fatigue Severity (PGIS).
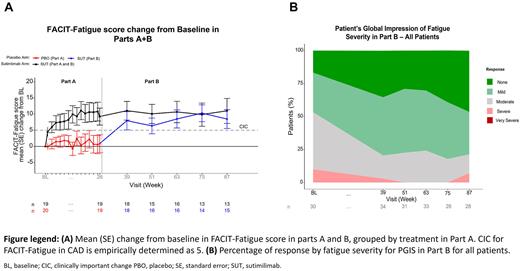
Results: Of the 42 patients enrolled in Part A of the study, 39 completed Part A and entered Part B, with 32 (82.1%) patients completing Part B. Patients randomized to sutimlimab in Part A showed a clinically meaningful improvement in FACIT-Fatigue mean score over baseline that exceeded the clinically important change (CIC) of 5 points by Week 1, and this was sustained at Week 87 (n=13), beyond one year after completing Part A (Figure Panel A). Patients who were randomized to placebo in Part A (ex-placebo) demonstrated rapid improvement in FACIT-Fatigue scores upon initiating sutimlimab treatment in Part B. In this group, mean (SE) change from baseline in FACIT-Fatigue score was 7.96 points (2.83, n=18) by their first assessment after switch to open-label treatment in Part B (13 weeks after sutimilimab initiation), and mean improvements were sustained above CIC for over one year from completion of Part A. Improvements in the physical (PCS) and mental (MCS) component scores of the SF-12 in patients receiving sutimlimab in Part A were also considered to be above the CICs, determined as changes above 3.9 and 2.8 points respectively. Improvements from baseline above CIC were sustained in the PCS through 87 weeks in all patients in Part B. In Part B, the MCS remained above CIC for all patients treated with sutimlimab in Part A; improvements to MCS among patients from the ex-placebo group were consistently improved from baseline and exceeded CIC at Week 75. The mean (SE) changes from baseline for all patients at 87 weeks for PCS and MCS were 7.2 (2.06, n=27) and 3.93 (1.92, n=27), respectively. EQ VAS showed a consistent increase from baseline in all patients with mean (SE) change from baseline at Week 87 of 15.57 (4.01, n=13) points. PGIC remained positive throughout the treatment period, with 93.3% of patients still reporting improvement from baseline at Week 87. PGIS improved from 46.7% reporting no or mild fatigue at baseline to 78.6% at Week 87 (Figure Panel B).
Conclusion: The PRO data in Part B demonstrated sustained benefits from sutimlimab for patient QoL beyond 26 weeks, and one year or more after initiation of sutimlimab. These findings demonstrate that continued inhibition of the classical complement pathway via treatment with sutimlimab results in meaningful long-term benefits to fatigue and patient-reported QoL, in addition to improving hematologic parameters in patients with CAD.
This study was registered at www.clinicaltrials.gov: NCT03347422.
Disclosures
Roeth:Novartis: Consultancy, Honoraria; Apellis Pharmaceuticals: Consultancy, Honoraria; Biocryst: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding. Broome:Sanofi: Honoraria, Other: Participation on a Data Safety Monitoring Board or Advisory Board , Research Funding; Alexion: Other: Participation on a Data Safety Monitoring Board or Advisory Board, Speakers Bureau; Argenx: Other: Participation on a Data Safety Monitoring Board or Advisory Board, Research Funding, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees; Rigel: Research Funding. Barcellini:Apellis: Honoraria; Alexion: Honoraria; Biocryst: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Momenta: Honoraria; Novartis: Honoraria; SOBI: Honoraria; Agios: Honoraria, Research Funding; Sanofi: Honoraria, Speakers Bureau. Jilma:Sanofi: Consultancy, Honoraria, Other: travel costs and lectures. Hill:Sanofi: Consultancy, Honoraria; Alexion: Honoraria; ReAlta: Honoraria; Sobi: Honoraria; Novartis: Consultancy, Honoraria; Janssen: Honoraria; Immunovant: Honoraria; Incyte: Honoraria; Amgen: Honoraria; Apellis: Consultancy, Honoraria; Argenx: Consultancy; Grifols: Consultancy, Honoraria. Cella:FACIT.org: Patents & Royalties: License fees, FACIT.org. Tvedt:Sobi: Other: Participation on a Data Safety Monitoring Board or Advisory Board ; Novartis: Other: Participation on a Data Safety Monitoring Board or Advisory Board ; Janssen: Other: Participation on a Data Safety Monitoring Board or Advisory Board. Murakhovskaya:Sanofi: Consultancy, Research Funding; Annexon: Research Funding; Kezar: Research Funding; Rigel: Consultancy, Research Funding; Alexion: Research Funding; Incyte: Research Funding; Novartis: Consultancy, Research Funding. Lee:Sanofi: Current Employment. Shafer:Sanofi: Current Employment. Wardecki:Sanofi: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: Planned filing of patent application concerning sutimlimab. Wang:Sanofi: Current Employment. Yoo:Sanofi: Current Employment, Current holder of stock options in a privately-held company. Mshijid:Sanofi: Current Employment, Current holder of stock options in a privately-held company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal